Methodology
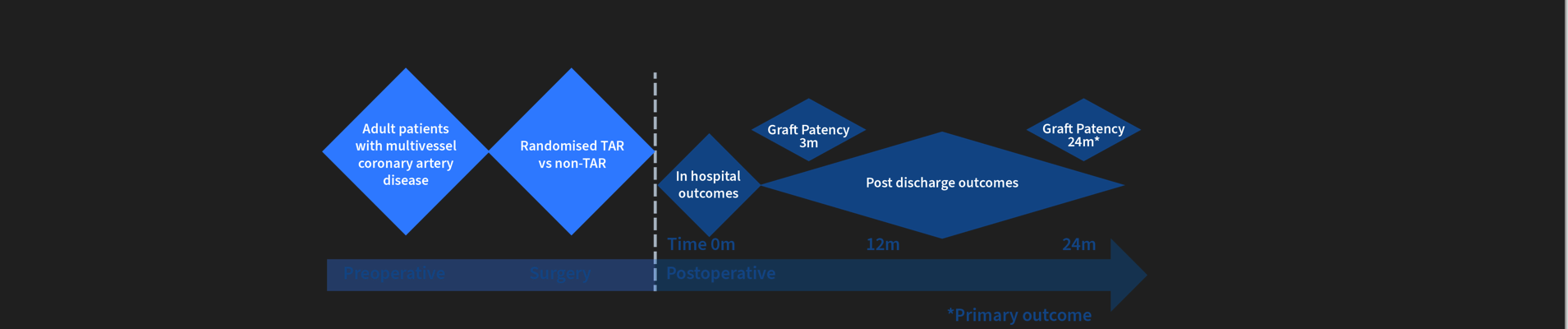
Trial Overview: Multicentre, prospective randomized clinical trial aimed at 17 study sites across Australia. This methodology was chosen to align with the MRFF aims of conducting large-scale projects definitively testing a new therapy to improve recovery and survivorship post cardiac events by better informing patients, clinicians and consumers. Patients will be randomised (1:1 allocation) to receive or not receive TAR during CABG surgery.
Trial oversight: The trial will be managed by the University of Melbourne (UoM) under the direction of the trial committee, with research support from the UoM. Melbourne Health will be Trial Sponsor.
Research question: Does the use of TAR improves angiographic graft patency, cardiac and cerebrovascular outcomes, and patient survival?
Interventional tool: Surgical technique comparing total arterial revascularisation (TAR) against non-TAR in patients undergoing primary isolated, non-emergent coronary artery bypass grafting (CABG) involving ≥2 grafts with wide inclusion criteria.
Participants: Primary isolated adult CABG patients with multivessel coronary disease requiring at least two grafts. Exclusion criteria include; previous cardiac surgery involving sternotomy; emergency operation, preoperative myocardial infarction within 48 hours; planned hybrid revascularisation; preoperative severe end-organ dysfunction (Troponin I > 20,000 within five days of surgery, ejection fraction < 20%, current inotrope or vasoconstrictor requirement, respiratory pressure support or intubation, acute renal failure or renal replacement therapy < 4 weeks, cerebrovascular event of any kind < 6 weeks before surgery, cancer or any comorbidity that reduces life expectancy to less than five years; planned concomitant procedure (including coronary endarterectomy and excepting for left atrial appendage occlusion or epicardial pacemaker lead placement); inability to participate in postoperative follow-up or CTCA including allergy to radiology contrast medium. These criteria are commensurate with the typical cardiac surgical patient and will reliably provide primary outcome study data.
Trial Design
Common management principles for study participants: For all patients, there is no trial stipulation as to conduit selection (other than TAR or not), revascularisation technique, use of sequential or composite methods or any perioperative management of any kind, including ICU management and any perioperative graft management in the short or long term, consistent with the participating surgeon’s usual practice. For patients randomised to the non-TAR group, ≥1 SVG must be used by any reconstructive method. In the case of use of the radial artery cannulated for coronary angiography, it is strongly recommended that ultrasound examination of RA is conducted before use, but that use of the RA is at the discretion of the surgeon. Optimal medical therapy is recommended for all patients after hospital discharge.
Trial Intervention
Group 1: Non-TAR: At least one SVG will be used without restricting the total number of SVG that can be used and applied to any coronary target. There is no degree of native coronary stenosis specified for any target coronary or any conduit.
Group 2: TAR: No SVG will be used. No specification as to arterial conduit use or reconstruction technique is made. There is no degree of native coronary stenosis specified for any target coronary or for any conduit.